Medical Properties of Cannabinoids
Cannabinoids are a set of about 80-100 compounds found only in cannabis plants. The cannabinoids are a subset of terpenoids.
Terpenoids are a very large class of naturally occurring organic chemicals that come in thousands of varieties. They contribute to scents and flavors and colors of plants, such as cinnamon or ginger. Many are valued for herbal or medicinal properties(antibacterials, analgesics), such as menthol, camphor, or eucalyptol.
Thus, all cannabinoids are terpenoids,
BUT not all terpenoids are cannabinoids.
Cannabis does produce many terpenoids that are not cannabinoids, as well, but the cannabinoids are currently the compounds in which most people are interested. Nevertheless, testing laboratories are embarking on a quest to identify and quantify several of the other terpenoids in cannabis that may, also, have medicinal qualities.
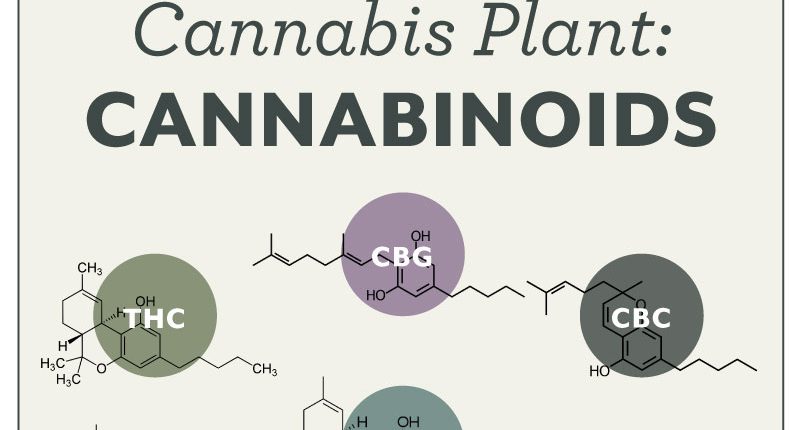
Cannabis plants are unique in that they make a compound called CBGA (cannabigerol acid). This compound is the “mother” or “grandmother” to all of the rest of the cannabinoids. Some of the more prevalent cannabinoids are listed below. In the plant, CBGA gets converted mostly along three major divisions:
- THCA and derivatives,
- CBDA and derivatives, and
- CBCA and derivatives.
Most of the currently common strains produce primarily the THCA family (THC Molecule shown at left).
Years of hybridization efforts to increase the psychoactive potency of cannabis have, largely, been successful in this.
A few strains are known to sometimes produce more of the CBDA family and are of great interest now due to recent successful studies showing the great potential of CBD, in particular, as effective for treating numerous ailments.
The CBCA family is much less studied at this point, but, with the advent of new technologies*, the identification of strains with significant amounts of the CBCA family is just beginning. In fact, this is true of almost all of the cannabinoids other than THC and CBD.
The research on most other compounds is still in its infancy, but results are being produced at an increasingly rapid pace.
Tests Available for These Common Cannabinoids*:
As always, consult your physician regarding the use of any medicine.
- “CBGA” = cannabigerol acid– CBGA is the primary cannabinoid from which all others derive. The plant first makes CBGA and then converts it, sometimes in several sequential steps, into the others. There are three major branches: (1) THCA and derivatives, (2) CBDA and derivatives, and (3) CBCA and derivatives. At certain stages of development, significant amounts of CBGA are observed in plant samples, but typically there is less than 2% at harvest. Like many of the acid forms of cannabinoids, it has been reported to have anti-inflammatory properties. A patent filed in 2006 also claimed analgesic properties and many others have also been reported.
- “CBG” = cannabigerol – When acid forms of cannabis are heated (or exposed to lots of UV light) they lose a molecule of carbon dioxide and form the neutral cannabinoid (sometimes called the phenol). CBG has been reported to have anti-proliferative (anti-tumor) and antibacterial activity, among others. CBG has been reported to inhibit growth of human epithelial cancer tumors.
- “THCA” =D9-THCA = Delta9-THCA A: D9-tetrahydrocannabinol acid – This is the precursor to THC and is typically the most abundant cannabinoid produced in most plants grown at present. (Dried cannabis typically contains 15-25% THCA.) A large fraction (but not all ) of the THCA converts to THC upon strong heating (> 200°F). The amount converted depends on the details of the temperature and timing. THCA has been shown to have anti-spasmodic and anti-proliferative (anti-cancer) properties, as well as evidence of anti-inflammatory activity. (In fact, there are other acid forms of THC, but they are almost always present in only very small quantities. THCA A and THCA B differ only in the placement of the carboxylic acid group. THCA A is almost always the version referred to when no designation is made.)
- “THC“= “D9-THC” = Delta9-THC = D9-tetrahydrocannabinol – Thought to be the most psychoactive of the cannabinoids and largely attributed with many beneficial medical properties, such as pain relief, appetite stimulation, anti-spasmodic properties, anti-emetic properties and many more. Plants don’t produce this compound directly. (Dried plant material contains only a few percent THC.) It is produced from THCA (see next entry) by heating or exposure to UV light. Not all THCA ends up as THC. Heating has been shown to convert at most about 70% of the THCA into THC. The “D9” in the name indicates the specific location of a carbon-carbon double-bond in one of the rings.
- “D8-THC” = Delta8–THC = D8-tetrahydrocannabinol – This compound has almost the exact same structure as D9-THC, except, as you might have guessed, the double bond in question is in the D8 position instead of the D9 position. Its effects are thought to be very similar to D9-THC. This compound is almost never produced in any significant amount by plants, but it is one of the few readily available standards that labs can purchase. Nevertheless, studies on the medicinal properties are few at this time.
- “THCA-C4” = Tetrahydrocannabinol-C4 – Version of THCA with a slightly shorter side chain. It only has a 4-carbon chain, whereas THCA has a five-carbon chain. Believed to have similar effects to THCA.
- “THVA” = tetrahydrocannabivarin acid – A version of THCA that only has three carbons (instead of 5) in the “alkyl side chain.” Believed to have similar effects to THCA.
- “THV” = “THCV” = tetrahydrocannabivarin – Formed when THVA is heated (or exposed to UV light). It has been reported to be anti-epileptic, anorectic, and a bone stimulant.
- “CBNA” = cannabinol acid – A breakdown product of THCA by air oxidation.
- “CBN” = cannabinol – Formed when CBNA is heated (or exposed to UV light).
- “CBDA” = cannabidiol acid – CBDA is at the top of the second major branch of CBGA derivatives and is thought to concentrate in the glandular hairs. It has been shown to exhibit anti-proliferative (anti-tumor) effects, among several other attributes. Some strains of cannabis occasionally yield plants that produce more CBDA than THCA, which, after heating, means more CBD than THC. Many patients prize these samples since they seem to exert many beneficial medical effects without the sometimes disorienting “high” associated with similar doses of THC. Most plant samples contain less than 2% CBDA. However, the few samples that do produce large amounts often contain over 10% CBDA (and only ~5% THCA).
- “CBD” = cannabidiol – CBD is, after THC, one of the most widely studied of the cannabinoids. It is believed to possess numerous beneficial medicinal effects. It has been clinically evaluated in anxiety, psychosis, and movement disorders, and to relieve neuropathic pain in patients with multiple sclerosis (in combination with D9-THC as a 1:1 mixture, i.e. Sativex). “Project CBD” is an organization that tracks the medical and cannabis industries for advances in the knowledge of CBD. They have many resources on their web site for learning more about CBD and its effects.
- “CBCA” = cannabichromene acid – CBCA sits at the top of the third major “daughter” branch of cannabinoids from CBGA. A recent patent claims that CBCA is produced primarily in the sessile trichomes of the plant (those without stalks). CBCA is thought to possess anti-inflammatory, antibacterial, and antifungal activity.
- “CBC” = cannabichromene – CBC forms when CBCA is heated (or exposed to UV light). Although one of the three most prevalent classes of cannabinoids, CBC has, to date, received relatively little attention. CBC is reported to exert anti-inflammatory, antimicrobial and modest analgesic activity. It also has been reported to be a bone stimulant.
- “CBLA” = cannabicyclol acid – CBLA is a degradation product. When CBCA absorbs UV light, CBLA is produced.
- “CBL” = cannabicyclol – CBL forms when CBLA is heated (or exposed to UV light).
Close Up line drawings of cannabinoid molecules (From Cannabinoid Science)
Original posting: Halent Technology
TriChome Imagery – Courtesy of Queen of Dragons | Shasta Lake, CA
 Want to start a cannabis business? Learn how you can start today! Click here!
Want to start a cannabis business? Learn how you can start today! Click here!